Medical Device Injection Molding at J&L
Tooling and Injection Molding Solutions for your Medical Device Project
J&L Plastic Molding will assist in proving your concept in the Prototype Tooling and Molding Stage. With the lessons learned in the prototype phase, J&L will meet your quality, tooling and molding needs in Production.
Medical Device Prototype Tooling
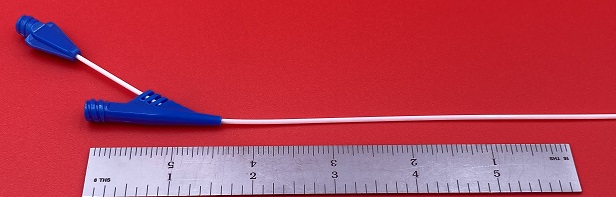
A successful prototype project for an injection molded component confirms part design, tests material selection, and proves the injection molding process in “production” conditions.
Use of Rapid Tooling Systems
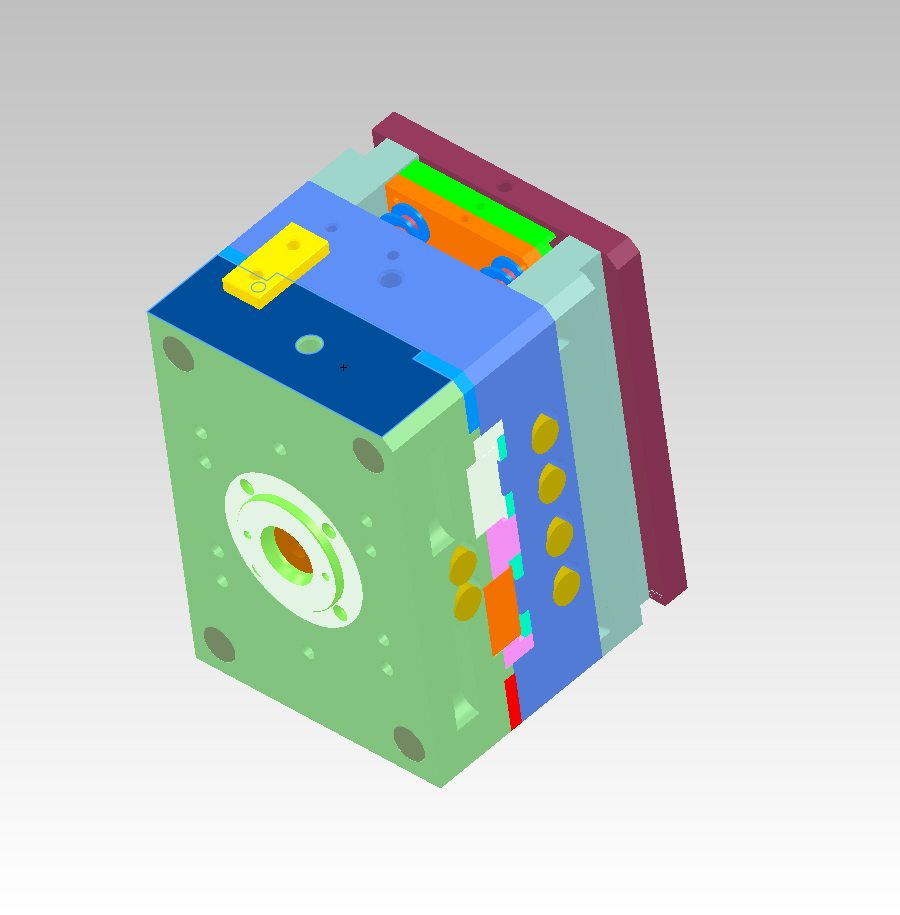
We use rapid tooling systems (MUD, Progressive and our own Prototype Plus) to replicate production conditions. SPOILER ALERT: many of our prototype tools transition to meet production requirements. We use steel mold components to simulate the demands placed on a production tool.
Steel Insert Blocks serve as Cavities
Steel Blocks serve as cavities and are inserted into the Rapid Tooling Frame. This saves money and time. It also allows for mold modifications during the prototype process. This system permits the molding process to replicate and test production processing. Processing lessons learned in the prototype phase will result in an efficient production tool.
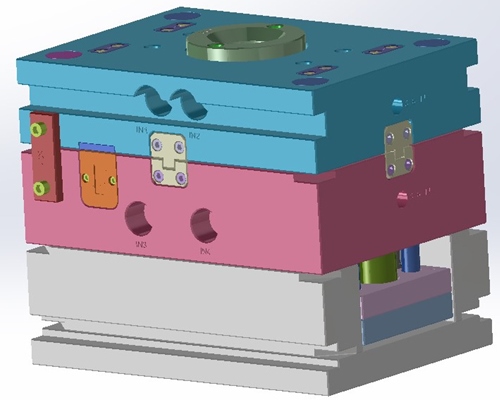
Medical Device Production Tooling
Based on a part’s final specifications and lessons learned during prototyping J&L builds Production Tools. Final Specifications must include an engineered drawing with tolerancing, 3D CAD Model, material selection, demand quantity, validation criteria and timing needs.
Medical Device Materials Processed at J&L
J&L has vast experience with engineering grades of material for medical device injection molding components. Here is a sampling of medical device material types and grades processed by J&L.
Radel R-5100 BK937 Black (PPSU)
Radel R-5100 is a popular material selection for medical device manufacturers to satisfy the increasing demand for re-usable devices. It will survive many cycles of many different sterilization methods.
| Manufacturer | Solvay |
| Material Details Link | Radel R-5100 |
Lustran 348 (ABS)
Lustran 348 is a high gloss medical grade ABS that meets U.S. Pharmacopeia 23 Class VI test requirements. It is well suited for medical device injection molding components.
| Manufacturer | INEOS Styrolution |
| Material Details Link | Lustran 348 |
Cycolac ABS HMG47MD
Cycolac HMG47MD is a biocompatible (ISO 10993) ABS grade. It is Gamma and EtO sterilizable.
| Manufacturer | Sabic |
| Material Details Link | Cycolac HMG47MD |
Makrolon 2558 (Polycarbonate)
Makrolon 2558 is medical device polycarbonate suitable for ETO and steam sterilization. It is available in Clear/Transparent and Opaque colors.
| Manufacturer | Covestro |
| Material Details Link | Makrolon 2558 |
Color Concentrate for Medical Devices
Finding the correct color solution for a new medical device component is challenging. Does it make sense to commit to a large minimum material buy of pre-colored resin before you’re ready to scale to production? If pre-colored material is too large an investment in the medical device’s development stage then color concentrates are an option. However, the risk on non-compliance of color concentrates must be managed.

A solution to the Color issue is Mevopur Healthcare Colorants from Avient. Mevopur colorants are available for use in commonly used and specialty grades of material. Its pre-tested for bio-compatibility and meets regulatory standards to minimize the risk of non-compliance. J&L will assist you with finding the correct material and color concentrate match.